What Are Pesticide Metabolites?
Question: I oftentimes hear about metabolites of pesticides. What are metabolites and what is their function in killing insect and mite pests?
Answer: A metabolite is a compound or substance formed during the process of chemical reactions (metabolism) by means of enzymes that occur in an organism or cell. Most pesticides are subject to metabolic degradation in plants or the environment. However, a number of currently available pesticides (insecticides and miticides) are converted into metabolites either inside an insect or mite pest, or within plant tissues.
Factors that can affect metabolism of certain insecticides and miticides include plant type and plant health. Insecticides and miticides that have activity against insect or mite pests as metabolites are referred to as pro-insecticides or pro-acaricides (miticides). Many insecticide and miticide metabolites may have no or very minimal insecticidal or miticidal activity due to detoxification or the metabolites are discarded from the insect or mite body during excretion. However, some processes may result in toxic metabolites.
In most cases, alterations to the active ingredient are required for some pesticides to confer toxic effects on insect and mite pests. For instance, most systemic insecticides are converted into metabolites that are more water soluble and are more active against insect pests.
In fact, all the neonicotinoid systemic insecticides registered for use in greenhouses that are applied to the growing medium as drench applications or granules are converted into metabolites. For example, when imidacloprid is applied to plants as a drench or granule, approximately 95 percent of the active ingredient (parent compound) is metabolized almost completely although this depends on the plant species.
The metabolites of imidacloprid are active against insect pests such as aphids. The primary metabolites of imidacloprid include olefine and 4-, and 5-dihydroxy. Olefine is 16 times more active on insect pests than imidacloprid because the metabolite has a higher attraction or affinity for the target site, nicotinic acetylcholine receptor, in certain aphid species. In addition, the water solubility of the metabolites is higher than the active ingredient. Thiamethoxam is converted into a metabolite that is actually another neonicotinoid systemic insecticide, clothiandin. Clothianidin is the least watersoluble of the neonicotinoid systemic insecticides at 327 ppm (327 mg/L) but has a higher binding affinity for the target site (nicotinic acetylcholine receptor). In addition, clothianidin is rapidly taken up in the transpiration stream and accumulates at higher concentrations in plant parts and tissues than other neonicotinoid systemic insecticides. Acetamiprid, which is another neonicotinoid systemic insecticide, but can only be applied to the foliage — not growing medium — is converted into five different metabolites. The metabolites of certain insecticides and miticides used in greenhouses are presented in Table 1.
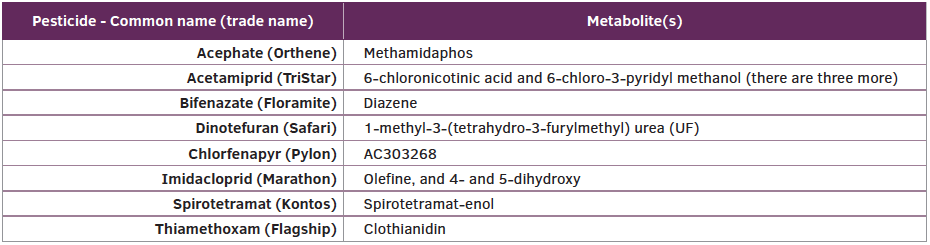
For more information on pesticide metabolites, refer to the extension publication, “Pesticide Metabolites,” available online at www.bookstore.ksre.ksu.edu/pubs/MF3070.pdf.


 Video Library
Video Library 




















