2019 Update: Bacterial Leaf Spots and Blight

The last time I reviewed bacterial disease control on ornamentals for GPN was in 2012, and a lot has happened in the ensuing years. There are new methods for identification, new diseases and several new effective bactericides, both conventional and biological in nature. This review will focus on foliar diseases that are caused by Acidovorax, Pseudomonas and Xanthomonas. Acidovorax species were primarily named Pseudomonas in the past and accounted for some of those that were classified as non-fluorescent Pseudomonads.
SYMPTOMS AND HOST RANGE
Leaf spots and foliar blights are the most common symptoms of diseases caused by Acidovorax, Pseudomonas and Xanthomonas. Small, water-soaked areas form initially on leaf edges, at stomates and at wounds occasionally. On some crops these spots are scattered all across the leaf, while on others they can be confined to leaf margins (Figure 1) or between leaf veins (Figure 2). If you find angular spots that form between leaf veins the chances that they are caused by bacteria are high.

Bacterial diseases are common in propagation of unrooted cuttings due to the addition of mist to facilitate rooting. Plants that grow better under dry conditions are especially stressed in propagation and become routinely infected with bacteria. Many similar symptoms can be caused by Pseudomonas and Xanthomonas on plants during propagation (Figure 3). Some propagators use Remay cloth to maintain high humidity with minimal water on the leaves.

Acidovorax and Pseudomonas are found in cool to hot climates depending on the exact species. When I worked in Florida, I saw three different bacteria causing leaf spots on hibiscus. Pseudomonas syringae occurred in the winter, Pseudomonas cichorii in the spring and fall and Xanthomonas was prevalent in the summer. The spots were very similar looking, but the bacteria causing them changed with the season. Since returning to the West Coast, I have only seen the Pseudomonas syringae in hibiscus from the West Coast hibiscus.
Generally speaking, Xanthomonas diseases are more often found in warm to hot climates. Xanthomonas diseases are often confined to a single genus or plant family. Xanthomonas on geranium is relatively specific to geranium while the Xanthomonas on dieffenbachia can attack anthurium, philodendron and really most members of the Aroid family. The Xanthomonas from zinnia is known to attack zinnia only. They used to be called pathogens of X. campestris but with the renaming frenzy, keeping up with what they are called now is challenging.
Pseudomonas diseases can be caused by a broad-host range species like P. cichorii, which attacks many tropicals, vegetables and other ornamentals. In the case of P. syringae pathovars, they can be broad-spectrum or narrow. Acidovorax species are somewhat specific on ornamentals often causing disease on a single genus.
DIAGNOSIS
Bacterial diseases remain the hardest to identify since fungi can be recognized by spores and selective media and viruses can be identified through antibody and related methods. Different labs utilize different methods to identify bacterial pathogens. Some use symptoms and research reports and other use bacterial steaming under a microscope and growth on bacterial media. Still others have access to sophisticated genetic fingerprinting technology. The most important thing to know is whether a foliar symptom is caused by bacterial pathogens or fungal pathogen or both. Table 1 shows the most common characteristics for phytotoxicity or fungal spots compared to those caused by bacteria.
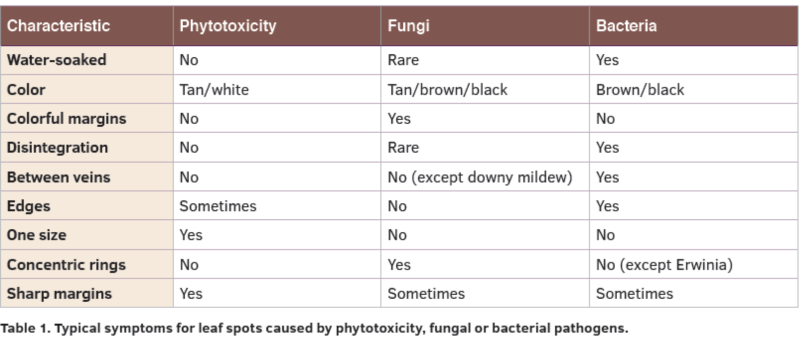
The only reason you would want to know which bacterium is involved is to make sure it is the cause of the symptom and then to consider all possible hosts in a spray program. Otherwise control strategies will be nearly identical for one bacterial disease or another.
One last point on bacterial diagnosis: just because you get a lab report that says a specific bacterium is the cause of your problem, it does not prove it is the cause. If it is a common disease then you are set. However, if it is new, ask for feedback from an expert from the university Extension service whenever possible.
CULTURAL CONTROLS
Generally, using cultural controls is not as effective for bacterial leaf spot diseases as for some other diseases like Botrytis blight and downy mildew. This is mainly due to the fact that the most important cultural control for foliar bacterial diseases is elimination of overhead watering and exposure to rainfall. Eliminating water on leaves will completely stop foliar bacteria from infecting. Since exposure to winter, spring or summer rainfall (depending on where you are in the U.S.) and use of overhead irrigation is so common in many operations this cultural control is rarely practical.
If you are producing crops from seed, you need to know that pathogenic bacteria like Xanthomonas (and also fungi like Alternaria) can be present in very, very low numbers. Zinnia is the best example of this and treating the seed with bleach has been very effective for over 30 years. The same is true for Xanthomonas diseases on stock (Matthiola), cabbage and other crucifers. Vegetable seeds like tomato and pepper also share at least one species of Xanthomonas and their seed is often pre-treated/cleaned to remove the pathogen from the outside of the seed. This type of treatment has not been routinely done for ornamental disease more or less due to the high value of the seed and the very small size of our industry.
RECENT DISEASE OUTBREAKS

In 2017, an outbreak of bacterial leaf stripe on canna was found in several nurseries in Florida (canna ‘Cannova Rose’ and ‘Cannova Bronze Scarlet’) and Texas (mixed colors of Cannova). Symptoms included dark-brown or black spots with a yellow halo that coalesced into necrotic stripes along leaf veins, especially on immature leaves (Figure 4). Acidovorax avenae subsp. avenae was identified as the cause of this new disease. Oregon State University reported the same pathogen on canna ‘South Pacific Scarlet’ from Washington State (2014). Leaf stripe was also recently reported causing leaf stripe on Strelitzia nicolai in Florida.
Begonias were first reported infected with Xanthomonas axonopodis pv. begonia (current name) in 1928 in Denmark. By 1938 it was well established in Europe and in 1939 the disease was reported in the United States. Since that time, Xanthomonas leaf spot and blight on begonias has been reported from nearly every region with occasional outbreaks. Despite some local folklore, all begonias (including Rex) are susceptible to this disease (Figure 5).

In late 2017 and early 2018, an outbreak of Xanthomonas blight on begonia occurred. Since little work (if any) has been reported on this disease since the late 1980s, we started trials comparing bactericides for safety and efficacy on begonias infected with Xanthomonas. The crops we used were: Begonia boliviensis ‘Bonfire Orange’, Begonia semperflorens-cultorum ‘Prelude Scarlet’, and Begonia tuber-hybrida ‘Nonstop Red’. Treatments were applied three times on a weekly interval and plants were inoculated after the first treatment. The treatments included:
A. Noninoculated control
B. Inoculated control
C. KleenGrow (6 oz/100 gal)
D. KleenGrow (12 oz/100 gal)
E. Kocide 3000 (32 oz/100 gal)
F. Camelot O (1 percent)
G. Phyton 27 (20 oz/100 gal)
Although the begonia species responded somewhat differently to the bactericides, it was clear that none of the products were 100 percent safe. The most sensitive species appeared to be the wax begonia but differences between the species were not significant. Overall, the 6-ounce rate of KleenGrow was somewhat safer than the three coppers but really none were safe enough for these begonia species to recommend their use. Disease severity was greatest on the ‘Bonfire Orange’ and least on the ‘Prelude Scarlet’. The best control across all begonia species in this trial was seen with the Phyton 27 and Camelot O.
BACTERICIDE TRIALS
Over 20 trials (mainly from Florida, California and Arizona) have been reported since 2012 on a variety of ornamentals for control of Pseudomonas or Xanthomonas (Table 2).
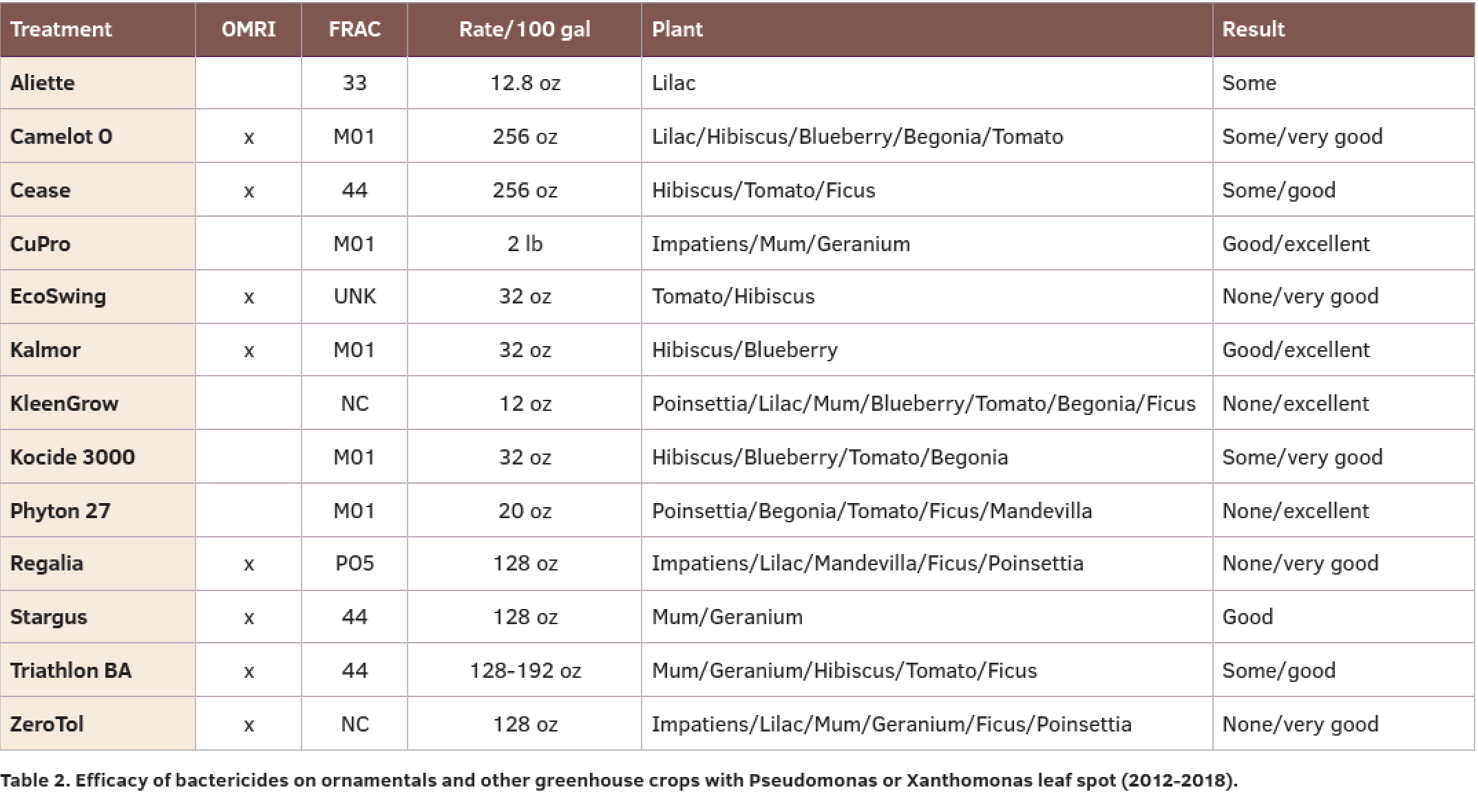
The copper products (FRAC M01) were overall effective for both Pseudomonas and Xanthomonas with a range of none to excellent control depending on plant and exact product used. Of this group, Camelot O and Kalmor are OMRI listed.
The plant extracts EcoSwing and Regalia are also OMRI listed and although tested in fewer trials during the 2012-2018 period, their results were significant in about 50 percent of the trials.
Bacillus-based products are classified as FRAC 44 and include Cease, Stargus and Triathlon BA. These products are not identical, as they have unique strains and showed a range from some to good control.
ZeroTol is not classified by FRAC and showed none to very good results in the six trials I located. Similarly, KleenGrow is not classified and in this case has shown none to excellent control in seven trials.
A bactericide rotation should be based on seven to 14-day intervals depending on growth rate of the crop and disease pressure. Bacterial leaf spots are very fast to develop due in part to the very short life cycle results in a population explosion in a few days. If you are growing an organic crop, you can use most of the products listed above.
CONCLUSIONS
Follow these steps to minimize losses from bacterial leaf spot diseases on ornamentals.
• Use only healthy cuttings, seedlings or liners.
• Do not overhead irrigate highly susceptible crops if possible, or irrigate when leaves will dry rapidly.
• Space plants to improve air circulation/drying time as well as bactericide coverage.
• Discard badly infected plants.
Get a lab diagnosis for diseases you do not easily recognize. Confusion between fungal and bacterial leaf spots is very easy and common. So are mixed infections with both types of pathogens. Rotate bactericides between different FRAC groups to limit possibility of resistance. Bacteria multiply very rapidly and are very prone to developing resistance to many products including copper and streptomycin sulfate.
Spray no more often than once a week. Spraying twice a week will be counter-productive and spraying more = MORE disease.
Follow product labels! The label is still the law.
A.R. Chase is plant pathologist at Chase Agricultural Consulting LLC and can be reached at archase@chaseresearch.net.


 Video Library
Video Library 




















