
Hydroponic Systems & Principles of Plant Nutrition
Hydroponic can be defined as growing plants in water containing nutrients. Examples of this type of hydroponic systems include NFT (nutrient film technique) systems and deep-water float systems where plant roots are set in nutrient solutions. Another definition of hydroponic is growing plants without soil. With this definition, growing plants in soilless media (potting soil) or other types of aggregate media such as sand, gravel, and coconut coir are considered hydroponic systems. Here, we are using hydroponics to mean growing plants without soil.
ESSENTIAL NUTRIENTS
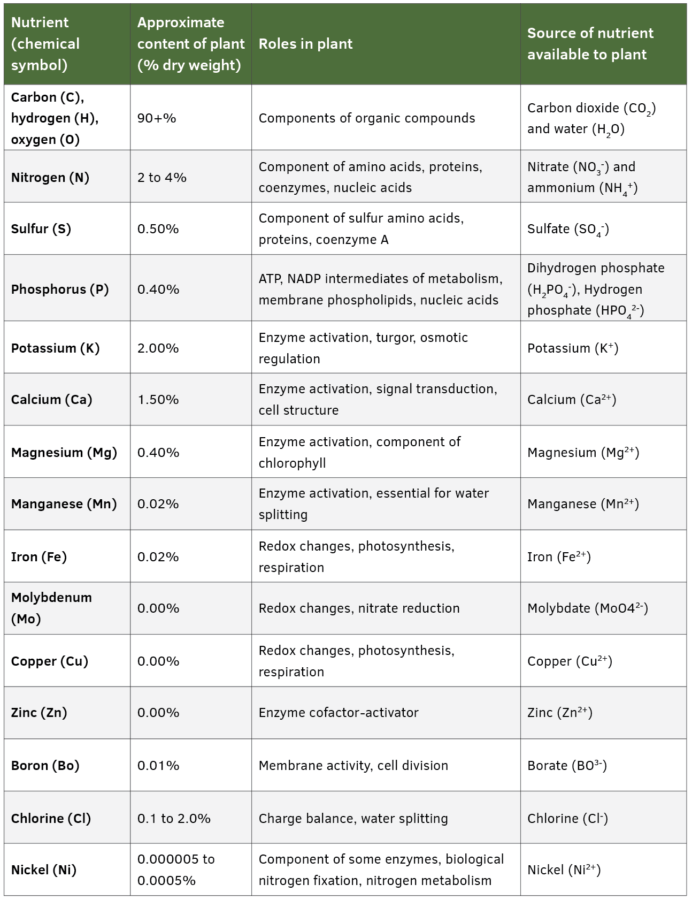
Plants cannot properly function without 17 essential nutrients. These nutrients are needed so that processes critical to plant growth and development can occur. For example, magnesium is a critical component of chlorophyll. Chlorophyll is a pigment used to capture energy from light that is needed in photosynthesis. It also reflects green wavelengths and is the reason most plants are green. Magnesium is the center of the chlorophyll molecule. Table 1 (below) lists the plant roles of essential nutrients.
Essential nutrients can be broadly categorized as macronutrients and micronutrients. Macronutrients and micronutrients are both essential for plant growth and development. Macronutrients include carbon, hydrogen, oxygen, nitrogen, phosphorus, potassium, sulfur, calcium, and magnesium. Micronutrients include iron, manganese, zinc, boron, molybdenum, chlorine, copper and nickel. The difference between macro- and micronutrients is the amount required by plants. Macronutrients are required in higher amounts than micronutrients. Table 1 shows the approximate plant content of essential nutrients.
Plants get carbon, hydrogen and oxygen from air and water. The rest of the nutrients are from soil or, in the case of hydroponics, from nutrient solutions or aggregate media. Sources of nutrients available to plants are listed in Table 1.
pH
It is impossible to discuss plant nutrition without considering pH. In hydroponics, we are primarily concerned with the pH of the water used to make up nutrient solutions and irrigate plants. pH is a measure of the relative acidity or hydrogen ion concentration, and it plays an important role in plant nutrient availability. It is measured using a 0- to 14-point scale where 0 is the most acidic, 7 is neutral, and 14 is the most alkaline. The scale is logarithmic, and each unit represents a 10-fold change. This means that small changes in values are large changes in pH. For example, a value of 7 is 10 times higher than 6 and 100 times higher than 5.
In general, the optimal pH range for growing vegetables hydroponically is 5.0 to 7.0.
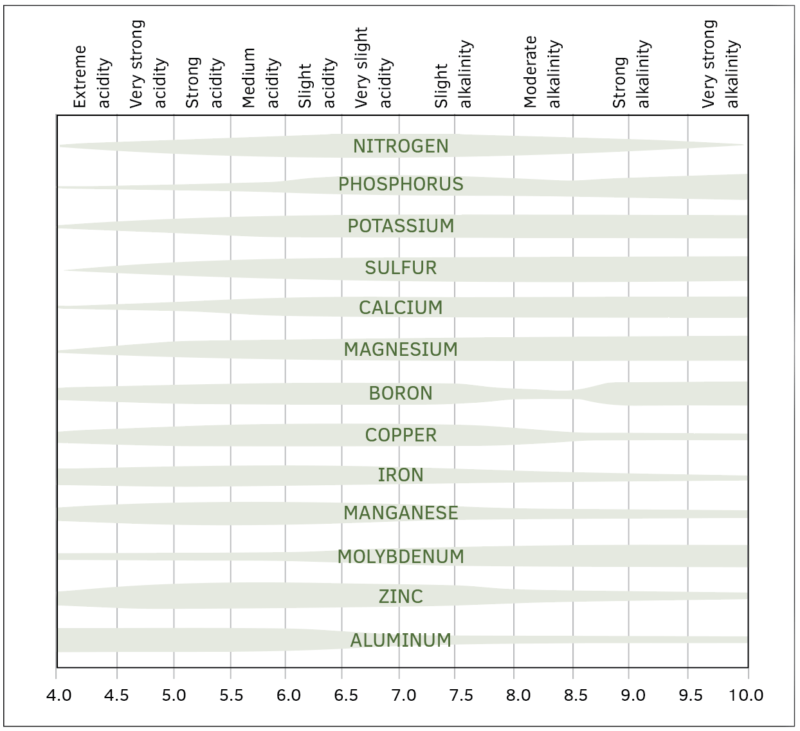
Figure 1 shows the relationship between nutrient availability and pH.
Along the bottom of the chart are different pH values between 4.0 and 10.0. At the top of the chart, the relative acidity or alkalinity is shown. Within the chart, relative nutrient availability
is represented by a bar. The wider the bar is, the more the nutrient is relatively available. For example, the nitrogen bar is widest between a pH of 6.0 to 7.5. This is the pH where it is most available to plants. It is very narrow between 4.0 and 4.5 and not as readily plant available.
It is also important to consider the alkalinity of the water. Alkalinity is a capacity measure — it measures the capacity of the water to neutralize the acid. This is due primarily to the combined amount of carbonate (CO3) and bicarbonate (HCO3), but hydroxide, ammonium, borate, silicate, and phosphate can also contribute.
When total alkalinity is low, the water has low buffering capacity. As a result, its pH will readily change depending on what is added to it. When total alkalinity is high, the pH of the water is high. Acid can be injected with irrigation water to decrease high pH water. The amount of acid needed depends on the alkalinity of the water.
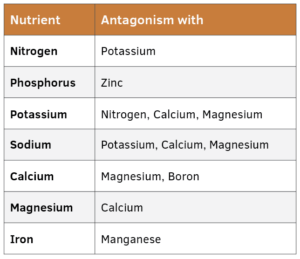
NUTRIENT ANTAGONISM AND INTERACTIONS
Nutrients are taken up by plants in roughly the same relative amounts that they are present in the nutrient solution. However, when one nutrient is in excess, it can be taken up at a higher amount at the expense of another nutrient. This is nutrient antagonism. In this case, it is possible to have sufficient amounts of a nutrient in the nutrient solution and for plants to be deficient.
For example, a recipe for a hydroponic tomato nutrient solution calls for 190 ppm nitrogen and 205 ppm potassium. Due to an error in calculating amounts of fertilizer to use, 2,050 ppm of potassium is added. Excess potassium in the solution can result in antagonism with nitrogen (and other nutrients) and can thus cause a nitrogen deficiency even if 190 ppm of nitrogen was added. Table 2 shows common antagonisms.
 NUTRITION PROBLEMS
NUTRITION PROBLEMS
Hydroponic systems are less forgiving than soil-based systems and nutrient problems can result in plant symptoms quickly. For that reason, the composition of the nutrient solution and regular monitoring of the nutrient solution and plant nutrient status is critical.
Additionally, keep an eye out for plant symptoms of common issues including:
Soluble Salts Damage
- Cause: Soluble salts damage can be caused by over-fertilization, poor water quality, salts accumulation in aggregate media over time and/or inadequate leaching. Fertilizers are salts and in hydroponic systems, they are most often fertigated. As water evaporates, soluble salts can accumulate in aggregate media if they are not adequately leached. Irrigation water can also be high in soluble salts contributing to the problem.
- Symptoms: Chemical induced drought can occur when soluble salts levels in planting media are excessive. As a result, you will see wilting of plants despite adequate irrigation. Other symptoms include dark green foliage, dead and burned leaf margins, and root death.
- Detection: Soluble salts levels can be monitored/measured by tracking the electrical conductivity (EC) of irrigation water, nutrient solutions and leachate (a nutrient solution that has drained from the planting container).
- Cure: Soluble salts can be leached with clear water. First, identify the source of the high soluble salts level and then correct it.
Nitrogen Deficiency
- Cause: Nitrogen deficiency can be caused by under fertilization, nutrient imbalance or excessive leaching.
- Symptoms: Typical first symptoms of nitrogen deficiency are light green foliage and overall stunting of plants. You can also see wilting and dead and/or yellow leaf margins.
- Detection: Measuring/monitoring the electrical conductivity (EC) of nutrient solutions can help prevent nitrogen deficiency. Adjust EC levels when they are low or high.
- Cure: Identify the source and correct it. This may mean adding more nitrogen to nutrient solutions. It may also mean that an antagonistic nutrient is excessive in the nutrient solution.
Calcium Deficiency
- Cause: Calcium deficiency can be caused by under fertilization, nutrient imbalance or low pH. It is also related to moisture management, high temperature and low airflow. Calcium is a mobile nutrient and is transported through the plant in water-conducting tissues. Fruit and leaves compete for water. Low relative humidity and high temperatures can result in increased transpiration rates and movement to leaves. In this scenario, calcium deficiency can develop in fruit.
- Symptoms: Calcium deficiency symptoms commonly start out as brown leaf margins of new plant growth or on the bottom of the fruit. Great examples of this are tipburn in lettuce and blossom end rot in tomato and pepper. As symptoms progress, you may see brown dead spots on the leaves.
- Detection: Monitor media and conduct plant analysis.
- Cure: Correct pH to between 5.0 and 7.0 in nutrient solutions. Apply fertilizers, if needed. In greenhouses, airflow can be low; introducing horizontal airflow at a rate of 0.3 to 1 m/s at plant level can break the plant boundary layer and increase the transpiration rate to avoid calcium deficiency in lettuce. The key with this is that airflow needs to be uniform for uniform plant growth.
Iron Deficiency
- Cause: The most common cause of iron deficiency is high pH in the media and/or irrigation water. It can also be caused by nutrient imbalance.
- Symptoms: Iron deficiency shows up in plants as yellowing between leaf veins. Look for this symptom to show up first on new growth.
- Detection: Monitor media and conduct plant analysis.
- Cure: Correct the pH of the nutrient solution. Apply iron fertilizer, if needed.
Magnesium Deficiency
- Cause: Magnesium can be caused by high media pH and/or nutrient imbalance.
- Symptoms: Look for yellowing between leaf veins as a symptom of magnesium deficiency. Magnesium deficiency usually shows up first on lower to middle leaves which helps distinguish it from iron deficiency.
- Detection: Monitor media and conduct plant analysis.
- Cure: Correct the pH of the nutrient solution. Apply iron fertilizer, if needed.
Boron Toxicity
- Cause: Boron toxicity is caused by applying too much boron to plants. Of the nutrients commonly applied as fertilizer, boron has the narrowest range between deficiency and toxicity. It is easy to over-apply boron. Check and double-check fertilizer calculations before applying. It can also be in irrigation water. It is important to check levels in a water source before using it and to account for boron in the water when adding boron fertilizer.
- Symptoms: Symptoms of boron toxicity are yellow and dead spots on leaf margins. You may also see reduced root growth.
- Detection: Monitor media and conduct plant analysis.
- Cure: Determine the source of the excess boron and correct it.


 Video Library
Video Library 




















